Pharmacometrics & Pharmacokinetics (PMK)
Poster Session I
PT-028 - CLINICAL ASSESSMENT OF THE DRUG INTERACTION POTENTIAL OF THE PSYCHOTROPIC NATURAL PRODUCT KRATOM (MITRAGYNA SPECIOSA).
Wednesday, March 22, 2023
5:00 PM - 6:30 PM EDT
R. Tanna1, J. Nguyen1, D. Hadi1,2, M. Layton1, J. White1, N. Cech3,2, N. Oberlies3,2, A. Rettie4,2, K. Thummel4,2, M. Paine1,2; 1Washington State University, Spokane, WA, USA, 2Center of Excellence for Natural Product Drug Interaction Research, Spokane, WA, USA, 3University of North Carolina at Greensboro, Greensboro, NC, USA, 4University of Washington, Seattle, WA, USA.

Rakshit S. Tanna (he/him/his)
PhD Candidate
Washington State University
Spokane, Washington, United States
Presenting Author(s)
Background: Calls to US poison centers involving kratom exposures increased 52-fold from 2011-2017, one-third of which reported use of oral kratom formulations with drugs of abuse. Many of these drugs are primarily eliminated through metabolism, particularly via CYP3A and CYP2D6, raising concerns for potential adverse pharmacokinetic kratom-drug interactions. Effects of kratom on midazolam (CYP3A probe) and dextromethorphan (CYP2D6 probe) were assessed in a powered clinical pharmacokinetic drug interaction study.
Methods: Twelve healthy adult volunteers participated in an open label, two-arm crossover, fixed sequence study. Midazolam (2.5 mg) and dextromethorphan (30 mg) were administered orally to obtain baseline pharmacokinetics. At least one week later, participants were administered the probe drugs 30 min after consuming a low dose (2 g) of the kratom product as a tea. Plasma pharmacokinetics (0-24 h) of the probe drugs were characterized via noncompartmental analysis. The geometric mean ratio (90% confidence interval) of the AUC, Cmax, and t1/2 in the presence to absence of kratom were determined.
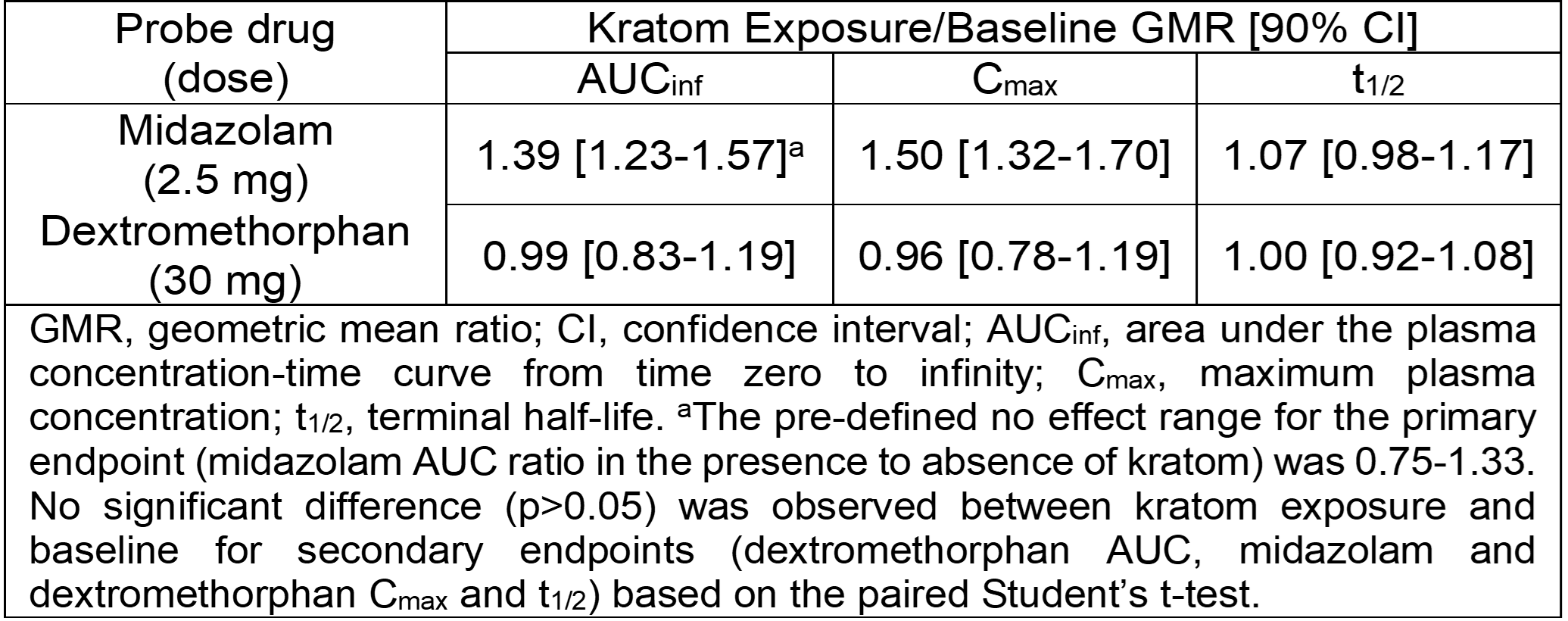
Results: Kratom altered midazolam but not dextromethorphan pharmacokinetics (see table)
Conclusion: This direct clinical evidence suggests that co-consuming kratom with other drugs extensively metabolized by CYP3A may precipitate serious interactions.