Back
Background: GBT021601 is a potent, next-generation HbS polymerization inhibitor with potential to be best-in-class for patients with sickle cell disease (SCD). Because GBT021601 has a terminal elimination half-life of ~10 days in patients with SCD, a modeling and simulation (M&S) approach was used to expedite clinical development by designing dosing regimens that quickly achieve steady-state (SS) concentrations and target percent hemoglobin (%Hb) occupancy (linked to efficacy). The adaptive M&S approach was considered in the design of studies in healthy volunteers (NCT05036512) and patients with SCD (NCT04983264, NCT05431088).
Methods: Single and multiple ascending dose pharmacokinetic (PK) and hematocrit data from healthy volunteers and patients with SCD were used to parameterize a combined population PK model of plasma and whole blood concentrations; the model was then used to simulate different loading- and maintenance-dose scenarios to achieve SS concentrations and target %Hb occupancy within 4 days of dosing.
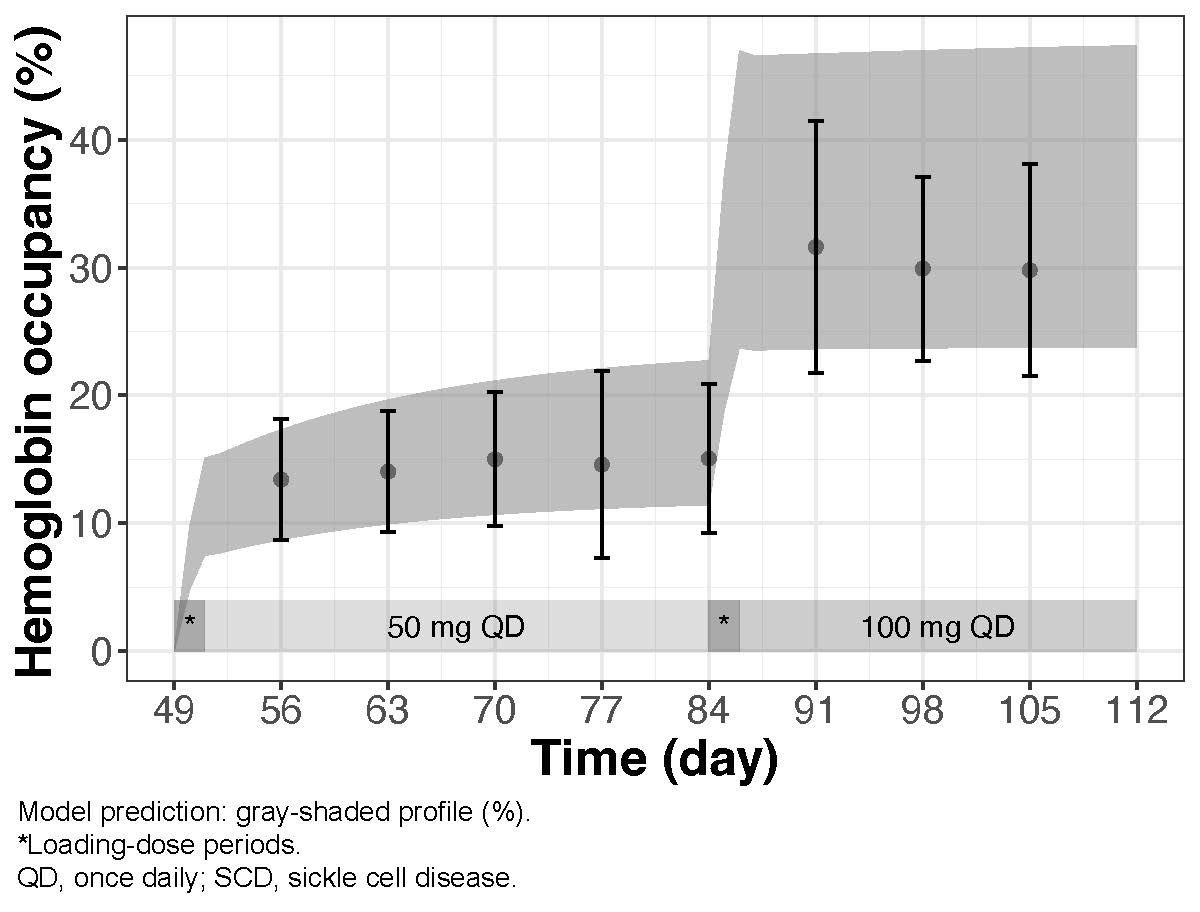
Results: The selected dose regimens achieved SS concentrations and %Hb occupancies within 1 week in patients with SCD (Figure) and healthy volunteers.
Conclusion: M&S was integral to the design and conduct of the clinical trials for GBT021601, allowing selection of dose regimens without prolonging the dosing duration or requiring additional cohorts.
Mean (SD) Observed Percent Hemoglobin Occupancy at Trough Compared With Model Predictions After Multiple-Dose Administration of 50 and 100 mg GBT021601 in Patients With SCD (NCT04983264)
Pharmacometrics & Pharmacokinetics (PMK)
Poster Walk II: Translational Approaches for Optimal Dosing In Patients
PWII-001 - MODEL-BASED APPROACH TO SELECT THE DOSE REGIMEN OF GBT021601, A NEXT-GENERATION SICKLE HEMOGLOBIN POLYMERIZATION INHIBITOR, IN HEALTHY VOLUNTEERS AND PATIENTS WITH SICKLE CELL DISEASE.
Wednesday, March 22, 2023
5:55 PM – 6:25 PM EDT
A. Lo1, A. Atluri2, T. Dumas2, K. Duchin1, E. Lisbon1, A. Barth1; 1Global Blood Therapeutics, South San Francisco, CA, USA, 2Certara, Princeton, NJ, USA.

Arthur Lo, PhD
Director - Modeling and Simulations/DMPK
Global Blood Therapeutics
South San Fransico, California, United States
Presenting Author(s)
Background: GBT021601 is a potent, next-generation HbS polymerization inhibitor with potential to be best-in-class for patients with sickle cell disease (SCD). Because GBT021601 has a terminal elimination half-life of ~10 days in patients with SCD, a modeling and simulation (M&S) approach was used to expedite clinical development by designing dosing regimens that quickly achieve steady-state (SS) concentrations and target percent hemoglobin (%Hb) occupancy (linked to efficacy). The adaptive M&S approach was considered in the design of studies in healthy volunteers (NCT05036512) and patients with SCD (NCT04983264, NCT05431088).
Methods: Single and multiple ascending dose pharmacokinetic (PK) and hematocrit data from healthy volunteers and patients with SCD were used to parameterize a combined population PK model of plasma and whole blood concentrations; the model was then used to simulate different loading- and maintenance-dose scenarios to achieve SS concentrations and target %Hb occupancy within 4 days of dosing.
Results: The selected dose regimens achieved SS concentrations and %Hb occupancies within 1 week in patients with SCD (Figure) and healthy volunteers.
Conclusion: M&S was integral to the design and conduct of the clinical trials for GBT021601, allowing selection of dose regimens without prolonging the dosing duration or requiring additional cohorts.
Mean (SD) Observed Percent Hemoglobin Occupancy at Trough Compared With Model Predictions After Multiple-Dose Administration of 50 and 100 mg GBT021601 in Patients With SCD (NCT04983264)


