Physiological Based Pharmacokinetic Modeling and Simulation (PBPK)
Poster Session I
PI-077 - DEVELOPMENT AND VERIFICATION OF A PYRIDOXIC ACID PBPK MODEL TO EVALUATE BIOMARKER INFORMED ORGANIC ANION TRANSPORTERS (OAT) 1 AND 3 INHIBITION.
Wednesday, March 22, 2023
5:00 PM - 6:30 PM EDT
S. Tan1, M. Willemin2, J. Snoeys2, H. Shen3, A. Rostami-Hodjegan1,4, D. Scotcher1, A. Galetin1; 1University of Manchester, Manchester, United Kingdom, 2Janssen Pharmaceuticals, Beerse, Belgium, 3Bristol Myers Squibb, Princeton, NJ, USA, 4Certara UK Limited (Simcyp Division), Sheffield, United Kingdom.

Shawn Pei Feng Tan
PhD Student
University of Manchester
MANCHESTER, England, United Kingdom
Presenting Author(s)
Background: Monitoring the levels of endogenous biomarkers in early clinical trials is increasingly used to evaluate transporter-mediated drug-drug interactions (DDIs). Pyridoxic acid (PDA) has been identified as the most sensitive plasma endogenous biomarker of renal OAT1/3 transporters in humans. The aim of this study was to develop and verify a physiologically-based pharmacokinetic (PBPK) model of PDA by leveraging published clinical DDI data with probenecid, a strong clinical OAT1/3 inhibitor.
Methods: PBPK model for PDA was developed using Simcyp V21. PDA biosynthesis rate and non-renal clearance were based on previous population pharmacokinetic modelling [1]. A top-down estimate of OAT1/3-mediated intrinsic clearance and experimentally measured passive diffusion clearance were used in the mechanistic kidney model. Probenecid PBPK model was adapted from the Simcyp database, modified, and re-verified to capture dose-dependent pharmacokinetics of probenecid (n = 5 studies).
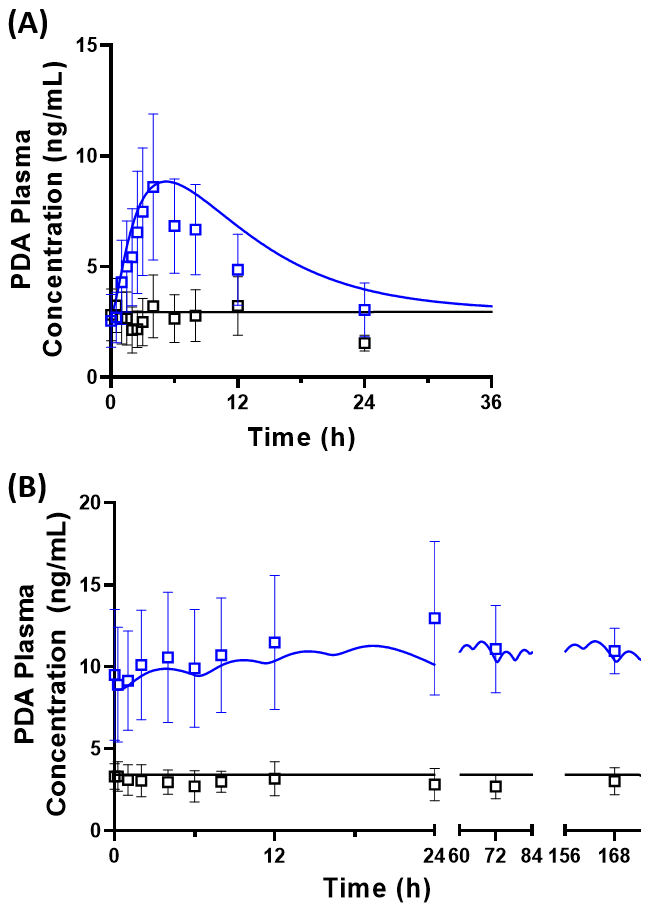
Results: See Figure 1.
Conclusion: The PDA PBPK model successfully predicted the extent of change in PDA concentrations and renal clearance after a DDI with either single or multiple doses of probenecid, highlighting its robustness. The verified PDA PBPK model is aimed to support future robust evaluation of the clinical relevance of OAT1/3 inhibition in drug development.
[1] Ahmad, A. et al. Population Pharmacokinetic Modeling and Simulation to Support Qualification of Pyridoxic Acid as Endogenous Biomarker of OAT1/3 Renal Transporters. CPT. Pharmacometrics Syst. Pharmacol. 10, 467-77 (2021).
[2] Shen, H. et al. Evidence for the Validity of Pyridoxic Acid (PDA) as a Plasma-Based Endogenous Probe for OAT1 and OAT3 Function in Healthy Subjects. J. Pharmacol. Exp. Ther. 368, 136-45 (2019).
[3] Willemin, M.E. et al. Clinical Investigation on Endogenous Biomarkers to Predict Strong OAT-Mediated Drug-Drug Interactions. Clin. Pharmacokinet. 60, 1187-99 (2021).
Figure 1. Predicted PDA plasma concentrations before and after a DDI with (A) 1.0 g single oral dose and (B) 0.5 g four times daily oral dose of probenecid. The solid line represents the predicted mean PDA plasma concentration. The symbols and error bars represent the mean ± standard deviation of observed PDA plasma concentrations from published clinical studies after single [2] and multiple probenecid doses [3]. Pre-and post-DDI phases are represented by the black and blue line/symbols, respectively. Predicted ratios of the area under the curve (post-DDI/pre-DDI) were within 1.5-fold of the observed data in both scenarios. The predicted percentage inhibition of PDA renal clearance was 63% and 79% after single and multiple dosing of probenecid, versus the observed values of 71% and 83%, respectively.