Back
Background: Copanlisib 60 mg IV on days 1, 8, and 15 of a 28-day cycle is approved in adult relapsed follicular lymphoma. PI3K-activation is implicated in pediatric cancers. We undertook PMX to support pediatric development.
Methods: PMX utilized physiologically based pharmacokinetic (PBPK) and population-based PK (popPK) modeling. An adult PBPK model was developed using physicochemical properties, mass balance, preclinical & clinical data to predict adult clinical PK. The qualified adult PBPK model was scaled to a virtual pediatric population (0.5 to 22 years) using age-dependent ADME to support starting doses for the pediatric clinical study conducted in collaboration with the Children’s Oncology Group (COG; Macy et al., ENA 2022). Lastly, an adult popPK model adapted with allometric scaling was used to propose the PK sampling scheme and to confirm tested doses in the pediatric study.
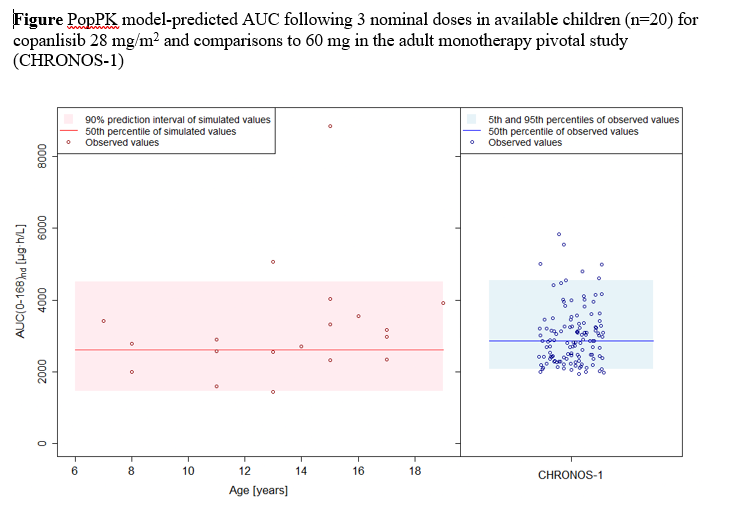
Results: The adult PBPK model successfully captured copanlisib clinical PK. The pediatric PBPK model supported the starting dose of 28 mg/m2 for children ≥ 1 year to exceed 80% of median adult AUC. The popPK model captured available clinical PK in pediatrics ≥ 7 years at 28 mg/m2 and 35 mg/m2 and the copanlisib RP2D of 28 mg/m2 achieved consistent AUC to adult 60 mg, confirming the PBPK model predictions (Figure).
Conclusion: A PMX framework supported and confirmed pediatric dosing for copanlisib.
Pharmacometrics & Pharmacokinetics (PMK)
Poster Session II
PII-075 - PHARMACOMETRICS (PMX) APPROACH TO SUPPORT PEDIATRIC DOSING FOR THE PAN-PI3K INHIBITOR COPANLISIB IN CHILDREN AND ADOLESCENTS WITH RELAPSED/REFRACTORY SOLID TUMORS.
Thursday, March 23, 2023
5:00 PM – 6:30 PM EDT
P. Morcos1, J. Schlender2, R. Burghaus3, J. Moss4, A. Lloyd4, F. Huang1, B. Childs1, M. Macy5, J. Reid6, J. Chung1, D. Garmann3; 1Bayer, Whippany, NJ, USA, 2Bayer AG, Leverkusen, Germany, 3Pharmaceuticals Division, Bayer AG, Wuppertal, Germany, 4BAST Inc. Limited, Leicester, United Kingdom, 5Children's Hospital Colorado, Aurora, CO, USA, 6Mayo Clinic, Rochester, MN, USA.

Peter N. Morcos, PharmD
Senior Director, Clinical Pharmacology
Bayer, United States
Presenting Author(s)
Background: Copanlisib 60 mg IV on days 1, 8, and 15 of a 28-day cycle is approved in adult relapsed follicular lymphoma. PI3K-activation is implicated in pediatric cancers. We undertook PMX to support pediatric development.
Methods: PMX utilized physiologically based pharmacokinetic (PBPK) and population-based PK (popPK) modeling. An adult PBPK model was developed using physicochemical properties, mass balance, preclinical & clinical data to predict adult clinical PK. The qualified adult PBPK model was scaled to a virtual pediatric population (0.5 to 22 years) using age-dependent ADME to support starting doses for the pediatric clinical study conducted in collaboration with the Children’s Oncology Group (COG; Macy et al., ENA 2022). Lastly, an adult popPK model adapted with allometric scaling was used to propose the PK sampling scheme and to confirm tested doses in the pediatric study.
Results: The adult PBPK model successfully captured copanlisib clinical PK. The pediatric PBPK model supported the starting dose of 28 mg/m2 for children ≥ 1 year to exceed 80% of median adult AUC. The popPK model captured available clinical PK in pediatrics ≥ 7 years at 28 mg/m2 and 35 mg/m2 and the copanlisib RP2D of 28 mg/m2 achieved consistent AUC to adult 60 mg, confirming the PBPK model predictions (Figure).
Conclusion: A PMX framework supported and confirmed pediatric dosing for copanlisib.

