Pharmacometrics & Pharmacokinetics (PMK)
Poster Session I
PI-055 - DOSE SELECTION FOR BRIGATINIB TO SUPPORT PEDIATRIC DEVELOPMENT.
Wednesday, March 22, 2023
5:00 PM - 6:30 PM EDT
T. Larson1, P. Diderichsen2, K. Venkatakrishnan1, N. Gupta1, M. Hanley1; 1Takeda Development Center Americas, Inc., Lexington, MA, USA, 2Certara, Princeton, NJ, USA.

Thomas R. Larson, PhD (he/him/his)
Clinical Pharmacologist
Takeda Development Center Americas, Inc.
Cambridge, Massachusetts, United States
Presenting Author(s)
Background: Brigatinib is a tyrosine kinase inhibitor approved for the treatment of ALK+ metastatic non-small cell lung cancer. The recommended adult dose is 180 mg QD with a 7-day lead-in at 90 mg QD. To support pediatric development, an adult population pharmacokinetic (PK) model was allometrically scaled to predict brigatinib PK in pediatric patients.
Methods: Pediatric dose projection was based on allometric scaling the adult model that described brigatinib PK using a 3-compartment model with first order elimination and a transit absorption compartment model. Virtual pediatric patients (aged 4-18 yrs) were simulated using the NHANES body size vs age distributions. The allometrically-scaled model was used to simulate pediatric exposures after administration of 3 dose levels of brigatinib. The simulated pediatric exposures were compared to the adult target exposure range at 90 and 180 mg QD to identify pediatric doses.
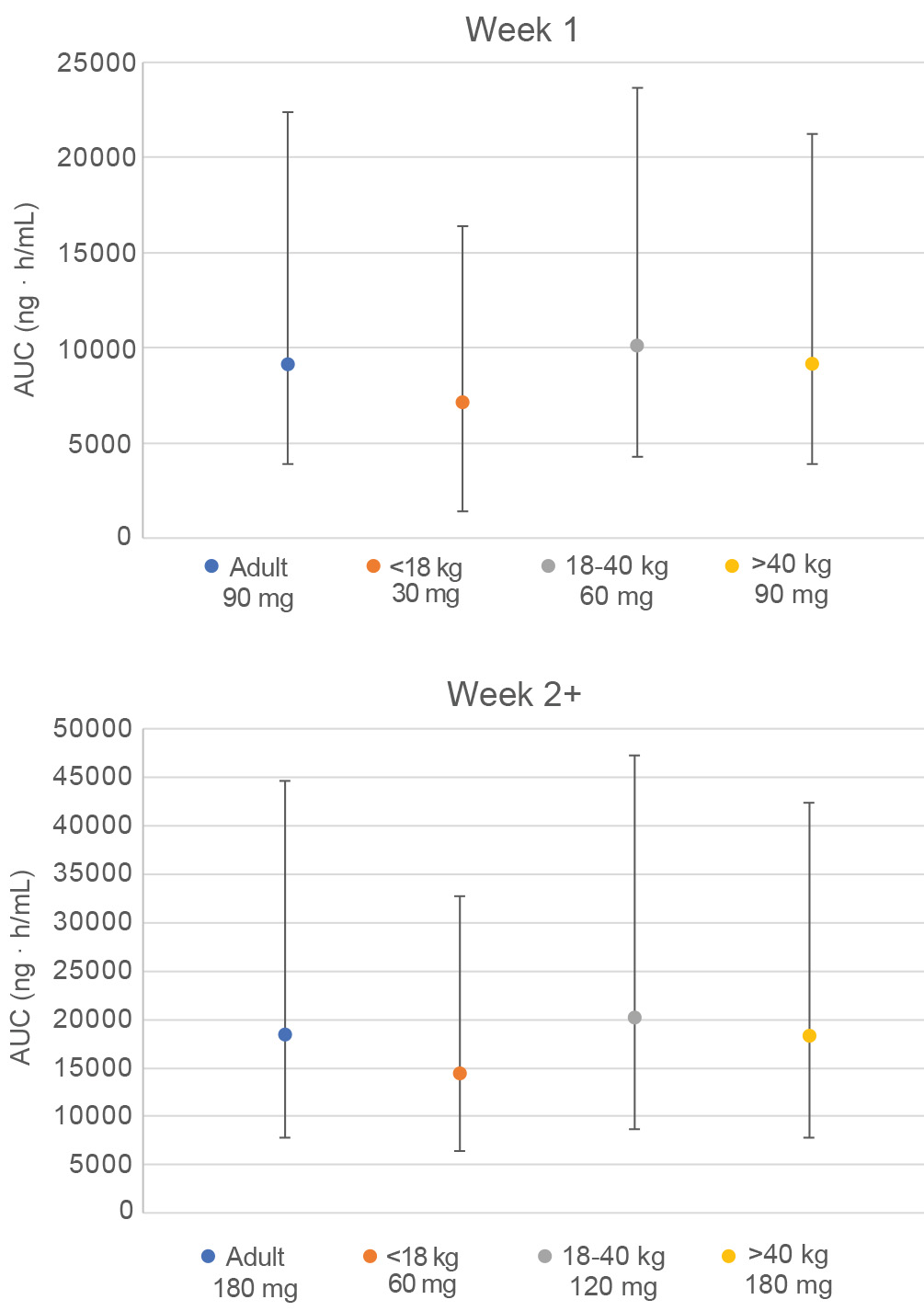
Results: Brigatinib doses of 30/60, 60/120, and 90/180 mg for pediatric patients < 18 kg, 18 to 40 kg, and >40 kg (respectively) were predicted to result in exposures that approximately match adult exposures at 90/180 mg QD (Figure).
Conclusion: The pediatric simulations support a brigatinib dosing approach using body weight bins in an ongoing Phase I/II pediatric study (NCT04925609).
Figure 1. Predicted Median and 5th to 95th Percentile of Brigatinib Area Under the Curve (AUC) in Each Pediatric Weight Bin and in Adult Patients Following Doses Selected to Match Adult Exposures at 90 mg QD (Week 1) to 180 mg QD (Week 2+)