Pharmacometrics & Pharmacokinetics (PMK)
Poster Session I
PI-058 - DRUG-DRUG INTERACTION POTENTIAL OF MAVACAMTEN WITH ORAL CONTRACEPTIVES: RESULTS FROM A CLINICAL PHARMACOKINETIC STUDY.
Wednesday, March 22, 2023
5:00 PM - 6:30 PM EDT
C. Manting, C. Sychterz, L. Gaohua, V. Perera, D. Gretler, V. Florea, S. Merali; Bristol Myers Squibb, Lawrenceville, NJ, USA.

Chiang Manting, PhD (she/her/hers)
Senior Scientist in Clinical Pharmacology & Pharmacometrics
Bristol Myers Squibb, United States
Presenting Author(s)
Background: Mavacamten is a potential inducer of cytochrome P450 (CYP) 3A4, and as such could reduce the exposure of the contraceptives, ethinyl estradiol (EE) and norethindrone (NOR), where CYP3A4 is involved in metabolism. This study assessed if repeat doses of mavacamten led to a drug-drug interaction with EE and/or NOR.
Methods: This was an open-label study in healthy women. Period 1: participants received 35 µg EE + 1 mg NOR. Period 2: participants received oral loading doses of mavacamten 25 mg on Days 1–2, 15 mg/day on Days 3‒17, and 35 μg EE + 1 mg NOR on Day 15. Plasma concentrations of mavacamten, EE and NOR were measured. A physiologically based pharmacokinetic (PBPK) model was used to simulate mavacamten-mediated CYP3A4 induction with EE for various CYP2C19 phenotypes.
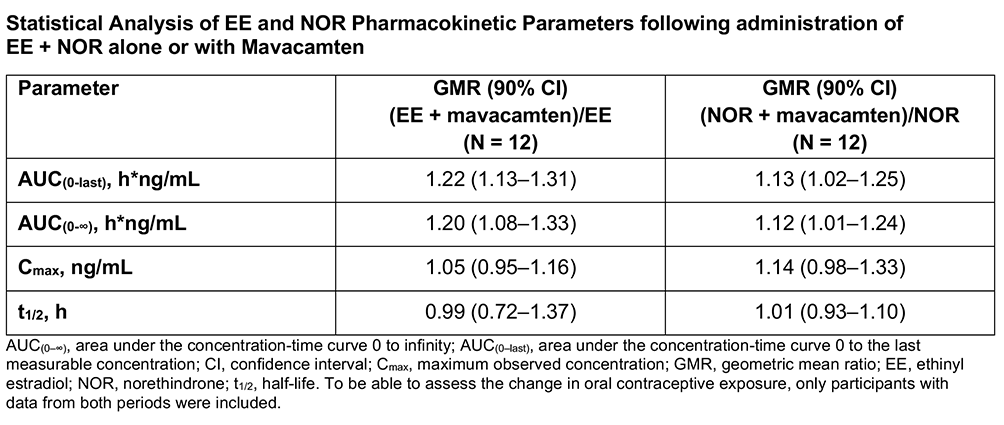
Results: In total, 13 women were enrolled (mean [SD] age: 38.9 [9.65] years). After mavacamten administration, modest increases in area under the concentration-time curves were observed for both EE and NOR; EE and NOR maximum concentrations and half-lives were not affected (Table). Criteria for bioequivalence were met or nearly met for EE and NOR exposure. Adverse events were mild. The PBPK model predicted a < 15% decrease in EE exposure across CYP2C19 phenotypes.
Conclusion: Mavacamten did not decrease the exposures of concomitantly administered EE or NOR.
Statistical Analysis of EE and NOR Pharmacokinetic Parameters following administration of EE + NOR alone or with Mavacamten