Oncology (ONC)
Poster Session I
PI-020 - IMPACT OF TYROSINE KINASE INHIBITOR (TKI) DOSAGE MODIFICATIONS ON PROGRESSION-FREE SURVIVAL (PFS) IN THE TREATMENT OF EGFR-MUTATED NON-SMALL CELL LUNG CANCER (NSCLC).
Wednesday, March 22, 2023
5:00 PM - 6:30 PM EDT
S. Carrasquilla, Y. Bi, W. Gu, A. Ayyoub, E. Pfuma Fletcher, J. Liu, A. Ramamoorthy, S. Shord, I. Zineh, R. Madabushi; US Food and Drug Administration, Silver Spring, MD, USA.

Santiago D. Carrasquilla, PhD
ORISE Postdoctoral Policy and Research Fellow
US Food and Drug Administration, United States
Presenting Author(s)
Background: Dosage modifications (DMs), such as reductions (DRs) and interruptions (DIs), are common in cancer treatment to manage drug toxicities. The impact of DMs on efficacy is not often known. We assessed whether DMs were associated with PFS for 4 FDA-approved anti-EGFR TKIs.
Methods: Patient-level data were collected from clinical trials supporting the original FDA approvals of afatinib, dacomitinib, mobocertinib, and osimertinib. In the analysis, DMs in patients were represented by the actual daily dose received (DDR; mg), with DIs represented as 0 mg. A Cox regression was performed with DDR as a time-varying covariate to assess the impact of DMs on PFS.
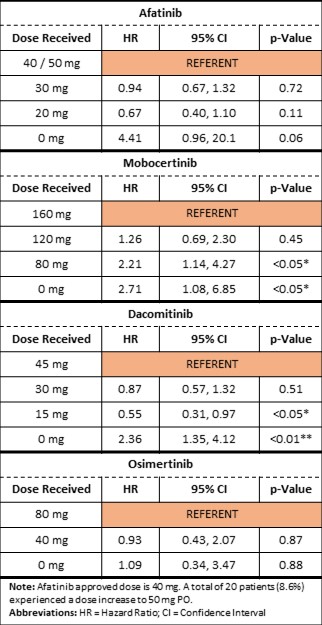
Results: DIs were associated with increased risk of progression for afatinib, mobocertinib, and dacomitinib (HR 2.4 - 4.4, p-value 0.06 - < 0.01). There was no clear association between DRs and PFS, except for mobocertinib for which an increased risk progression became apparent at a DR of 50% (Table 1).
Conclusion: DMs are often used in cancer treatment to mitigate adverse reactions. Our analysis suggests that, in general, DRs do not increase the risk of progression for patients receiving certain anti-EGFR TKIs for NSCLC. DIs, however, were significantly associated with worse outcomes. Further study for other TKIs and cancers is needed to assess the robustness and generalizability of these findings.
Time-Dependent Cox Regression to Assess the Impact on PFS