Back
Background: Quizartinib is a selective FLT3 inhibitor and a potential therapy for FLT3-ITD positive acute myeloid leukemia. The objective of this study was to determine the absolute oral bioavailability of quizartinib using an IV microdose of 14C-quizartinib in healthy subjects.
Methods: This was an open-label study in which healthy subjects received a single 60-mg oral dose (2×30-mg tablets) followed by a single IV infusion of 14C-quizartinib 50 µg containing not more than 14C 22.84 kBq 4 hours after oral dose (NCT04796831). Blood samples were taken before dose and at regular intervals until Day 22 post dosing. 14C-quizartinib was analyzed by accelerator mass spectrometry (AMS). Absolute bioavailability was calculated using AUClast and AUCinf for quizartinib after oral and IV administrations.
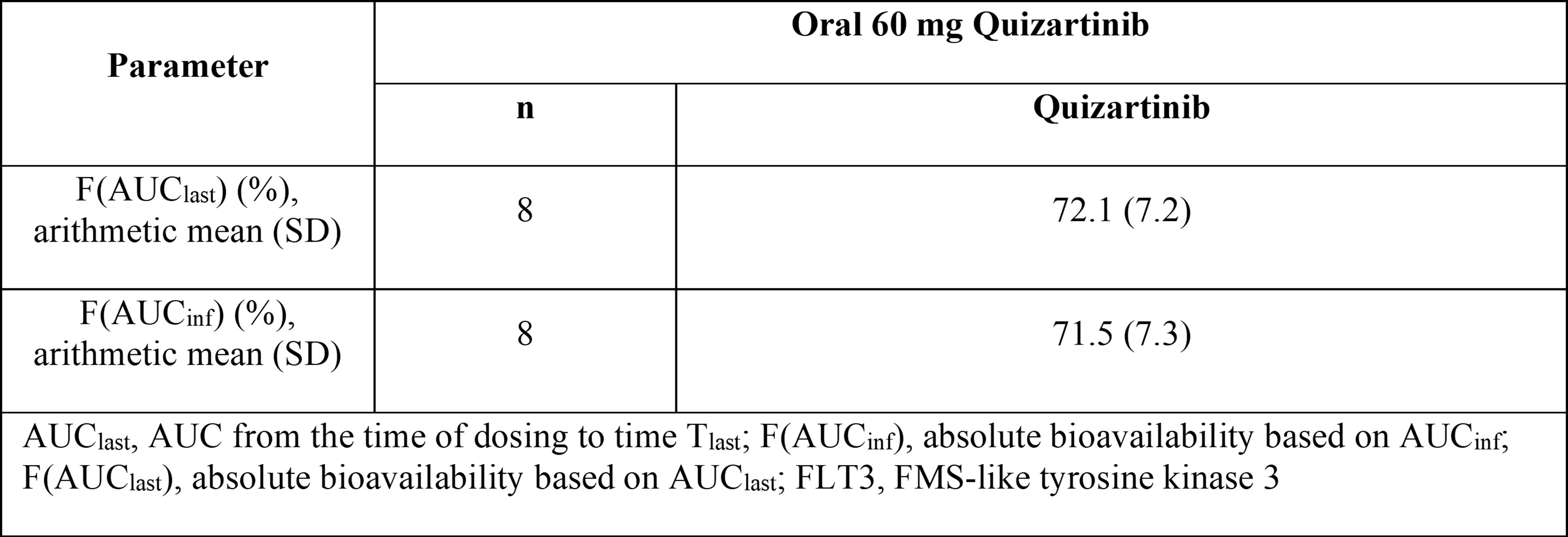
Results: All subjects (8/8) were white men; mean (SD) age was 35 (9) years. Mean (SD) absolute oral bioavailability of plasma quizartinib was approximately 71% (7%) based on AUCinf (Table 1).
Conclusion: Quizartinib absolute oral bioavailability from the final commercial tablet formulation was approximately 71%.
Table 1. Pharmacokinetic
Results: Plasma Quizartinib
Oncology (ONC)
Poster Session I
PI-014 - DETERMINATION OF ABSOLUTE BIOAVAILABILITY OF QUIZARTINIB USING A MICROTRACER.
Wednesday, March 22, 2023
5:00 PM – 6:30 PM EDT
H. Zahir1, N. Singh2, Y. Bermudez1, Y. Mostafa Kamel1, N. Said1, C. Hsu1, M. Abutarif1, M. Zheng1; 1Daiichi Sankyo, Basking Ridge, NJ, USA, 2Quotient Sciences, Nottingham, United Kingdom.
.jpg)
Ming Zheng, PhD
Daiichi Sankyo, United States
Presenting Author(s)
Background: Quizartinib is a selective FLT3 inhibitor and a potential therapy for FLT3-ITD positive acute myeloid leukemia. The objective of this study was to determine the absolute oral bioavailability of quizartinib using an IV microdose of 14C-quizartinib in healthy subjects.
Methods: This was an open-label study in which healthy subjects received a single 60-mg oral dose (2×30-mg tablets) followed by a single IV infusion of 14C-quizartinib 50 µg containing not more than 14C 22.84 kBq 4 hours after oral dose (NCT04796831). Blood samples were taken before dose and at regular intervals until Day 22 post dosing. 14C-quizartinib was analyzed by accelerator mass spectrometry (AMS). Absolute bioavailability was calculated using AUClast and AUCinf for quizartinib after oral and IV administrations.
Results: All subjects (8/8) were white men; mean (SD) age was 35 (9) years. Mean (SD) absolute oral bioavailability of plasma quizartinib was approximately 71% (7%) based on AUCinf (Table 1).
Conclusion: Quizartinib absolute oral bioavailability from the final commercial tablet formulation was approximately 71%.
Table 1. Pharmacokinetic
Results: Plasma Quizartinib

