Oncology (ONC)
Poster Session I
PI-016 - EFFECT OF MILD AND MODERATE HEPATIC IMPAIRMENT ON QUIZARTINIB PHARMACOKINETICS.
Wednesday, March 22, 2023
5:00 PM - 6:30 PM EDT
H. Zahir1, K. Lasseter2, T. Marbury3, J. Rondon2, Y. Bermudez1, G. Gammon1, Y. Mostafa Kamel1, N. Said1, C. Hsu1, M. Abutarif1, M. Zheng1; 1Daiichi Sankyo, Basking Ridge, NJ, USA, 2Clinical Pharmacology of Miami, Miami, FL, USA, 3Orlando Clinical Research Center, Orlando, FL, USA.

Malaz Abutarif, PhD
VP, Global Head of Quantitative and Clinical Pharmacology
Daiichi Sankyo, United States
Presenting Author(s)
Background: Quizartinib (Q) is a receptor tyrosine kinase FLT3 inhibitor and a potential therapy for FLT3-ITD AML. Two phase 1, open-label studies (NCT04473664) were conducted to determine the effects of mild or moderate hepatic impairment (HI; based on Child-Pugh [CP] or National Cancer Institute-Organ Dysfunction Working Group criteria [NCI-ODWG]) on the pharmacokinetics (PK) of Q and its active metabolite AC886 compared with matched healthy controls (MHC).
Methods: Blood samples were collected before administration of a single 30-mg Q tablet on day 1, then afterward on days 1-21. The effect of mild or moderate HI on the PK of Q and AC886 were assessed by comparing Cmax, AUClast, and AUCinf in the HI subjects vs MHC using ANOVA.
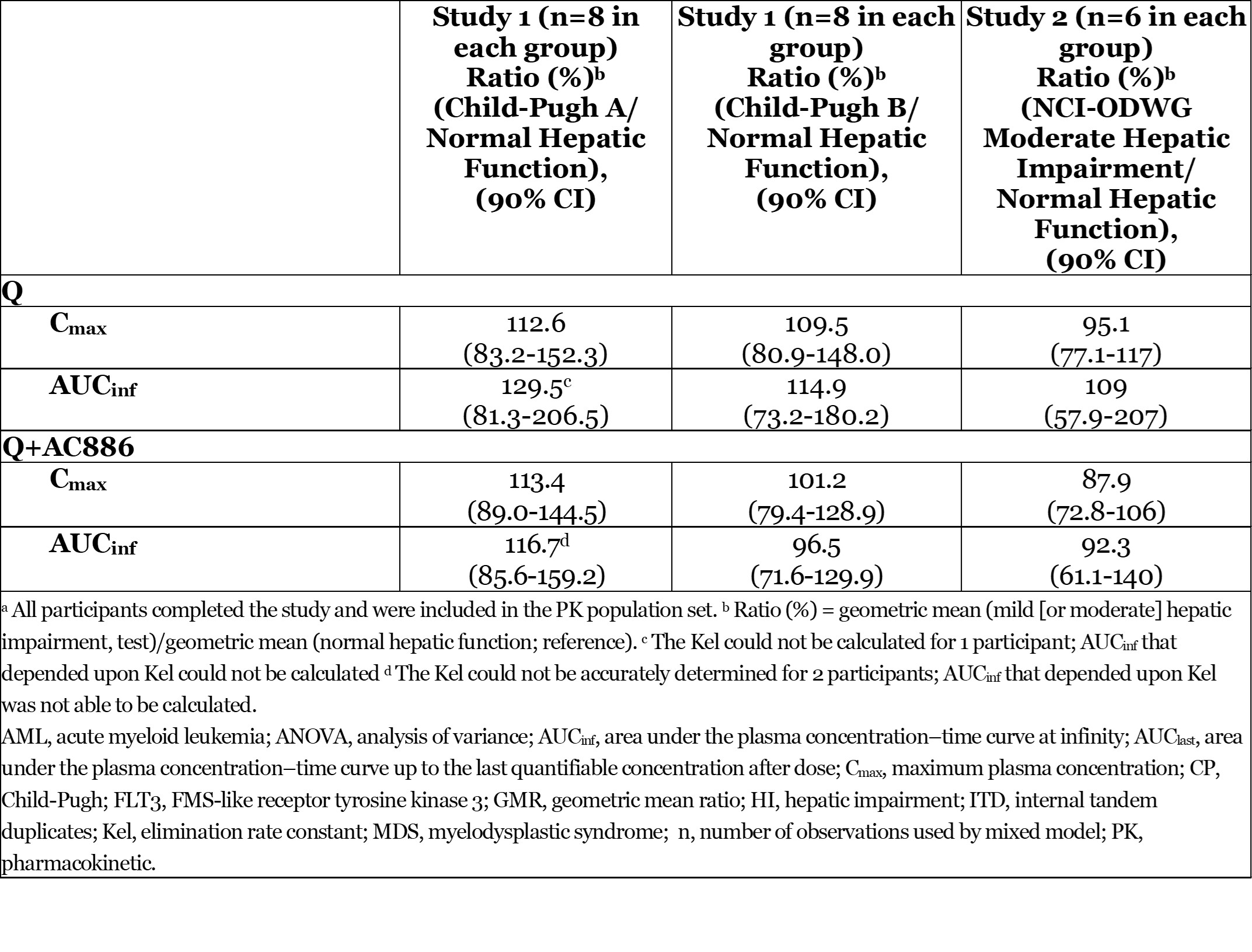
Results: Subjects with mild HI (CP-A, n=8), moderate HI (CP-B, n=8; NCI-ODWG, n=6), and MHC (CP, n=14; NCI-ODWG, n=6) were enrolled and completed the studies. The effects of mild and moderate HI on Q PK are shown (Table). Q mean T1/2 was longer in CP-A (119h) and CP-B subjects (114h) vs MHC (88h) but was similar in NCI-ODWG moderate HI subjects (93.7h) and MHC (96.3h). Q was well tolerated.
Conclusion: Moderate HI was not associated with increase in Q exposure. Differences were not considered clinically meaningful. No dose adjustment of Q is required in patients with mild or moderate HI.
Table. Effect of Hepatic Impairment on Q and AC886 PK After Administration of Q 30 mg Tablet in Subjects With Mild (Child-Pugh A) or Moderate (Child-Pugh B or NCI-ODWG) Hepatic Impairment and MHC: ANOVA Modela