Back
Background: Mitapivat, a first-in-class, oral, small molecule, allosteric activator of the red blood cell pyruvate kinase (PK) enzyme, is approved in the US for the treatment of hemolytic anemia in adults with PK deficiency. Here, we present a population pharmacokinetic analysis to identify candidate doses for evaluation in the phase 3 studies in pediatric subjects with PK deficiency.
Methods: A three compartment pharmacokinetic model previously developed in adults was adopted, with clearance and volume of distribution parameters allometrically scaled according to body weight. Maturation factors accounting for the changes in CYP3A activity were considered for subjects aged 1 to < 2 years. Dose simulations were conducted using a virtual pediatric population based on the NHANES database.
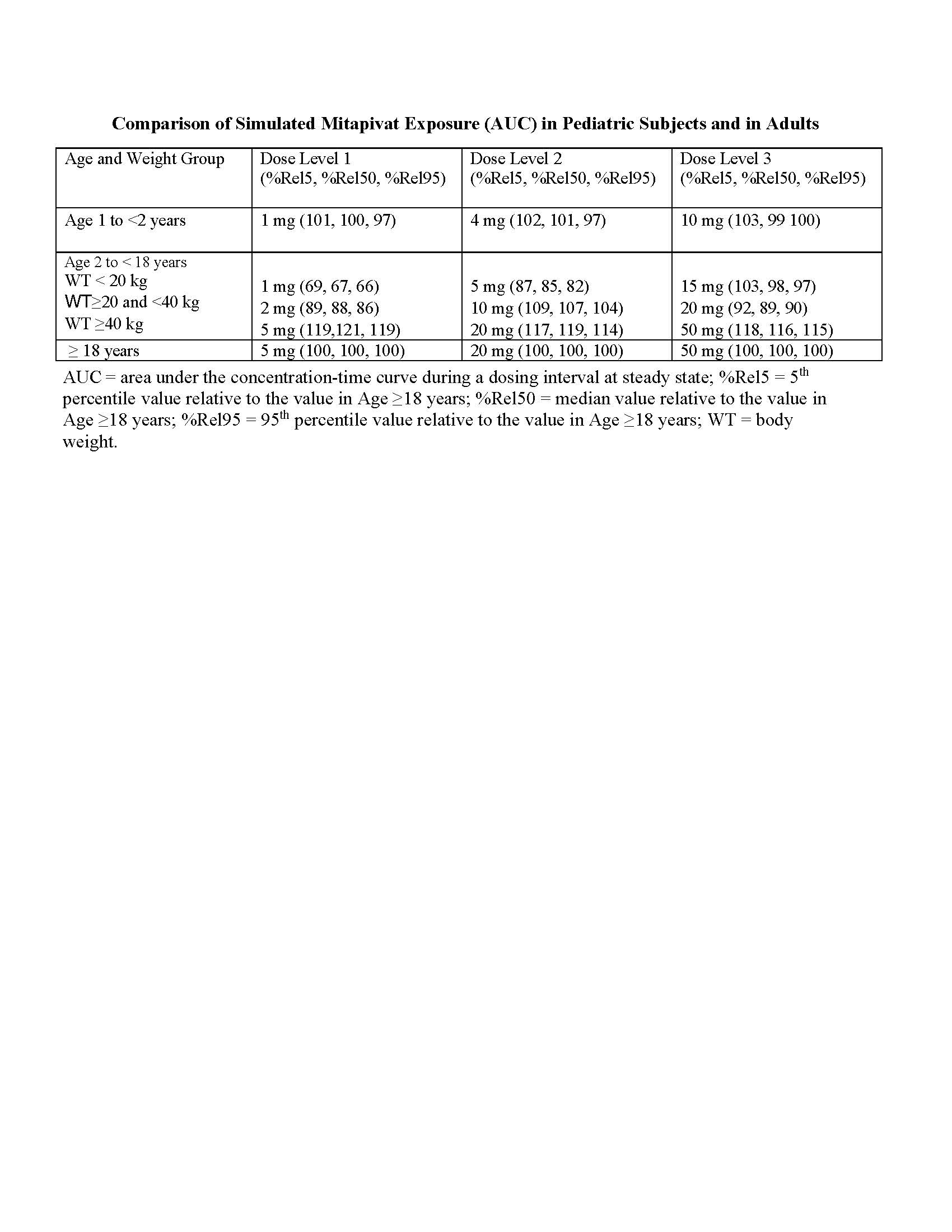
Results: Proposed dosing scheme and the corresponding model-predicted mitapivat exposure are shown in the Table. The selected pediatric doses for each age subset and weight range are predicted to achieve mitapivat AUC similar to that achieved in adults receiving the recommended clinical dose for PK deficiency; overall the predicted difference in AUC is < 20%.
Conclusion: Population pharmacokinetic modeling and simulation supported dose selection in mitapivat pediatric phase 3 studies.
Comparison of Simulated Mitapivat Exposure (AUC) in Pediatric Subjects and in Adults
Pharmacometrics & Pharmacokinetics (PMK)
Poster Session I
PI-056 - DOSE SELECTION FOR MITAPIVAT PEDIATRIC PHASE 3 STUDIES USING POPULATION PHARMACOKINETIC MODELING AND SIMULATION.
Wednesday, March 22, 2023
5:00 PM – 6:30 PM EDT
O. Yin1, K. Baron2, J. Mondick2, M. Little1, P. Tyler1, V. Beynon1; 1Agios Pharmaceuticals, Inc., Cambridge, MA, USA, 2Metrum Research Group, Tariffville, CT, USA.

Ophelia Q. Yin, PhD
Head of Pharmacometrics
Agios Pharmaceuticals, Inc.
Westfield, New Jersey, United States
Presenting Author(s)
Background: Mitapivat, a first-in-class, oral, small molecule, allosteric activator of the red blood cell pyruvate kinase (PK) enzyme, is approved in the US for the treatment of hemolytic anemia in adults with PK deficiency. Here, we present a population pharmacokinetic analysis to identify candidate doses for evaluation in the phase 3 studies in pediatric subjects with PK deficiency.
Methods: A three compartment pharmacokinetic model previously developed in adults was adopted, with clearance and volume of distribution parameters allometrically scaled according to body weight. Maturation factors accounting for the changes in CYP3A activity were considered for subjects aged 1 to < 2 years. Dose simulations were conducted using a virtual pediatric population based on the NHANES database.
Results: Proposed dosing scheme and the corresponding model-predicted mitapivat exposure are shown in the Table. The selected pediatric doses for each age subset and weight range are predicted to achieve mitapivat AUC similar to that achieved in adults receiving the recommended clinical dose for PK deficiency; overall the predicted difference in AUC is < 20%.
Conclusion: Population pharmacokinetic modeling and simulation supported dose selection in mitapivat pediatric phase 3 studies.
Comparison of Simulated Mitapivat Exposure (AUC) in Pediatric Subjects and in Adults



