Pharmacometrics & Pharmacokinetics (PMK)
Poster Session I
PI-042 - BUDESONIDE EMBEDDED ALGINATE THIN FILMS SHOW TUNABLE SUSTAINED RELEASE.
Wednesday, March 22, 2023
5:00 PM - 6:30 PM EDT
R. Chevalier, N. Almahbub, T. Lin; Children's Mercy Kansas City, Kansas City, MO, USA.

Rachel Chevalier, MD (she/her/hers)
Assistant Professor
Children's Mercy Kansas City
Leawood, Kansas, United States
Presenting Author(s)
Background: Eosinophilic esophagitis leads to dysphagia and food impaction. Oral viscous budesonide is the pharmacologic treatment of choice; however, budesonide treatment fails in 25-35% of patients. Esophageal delivery is complicated by gravity, peristalsis, and poor absorption. We are designing a mucoadhesive, high aspect ratio thin film-based dispersible formulation of budesonide for EoE.
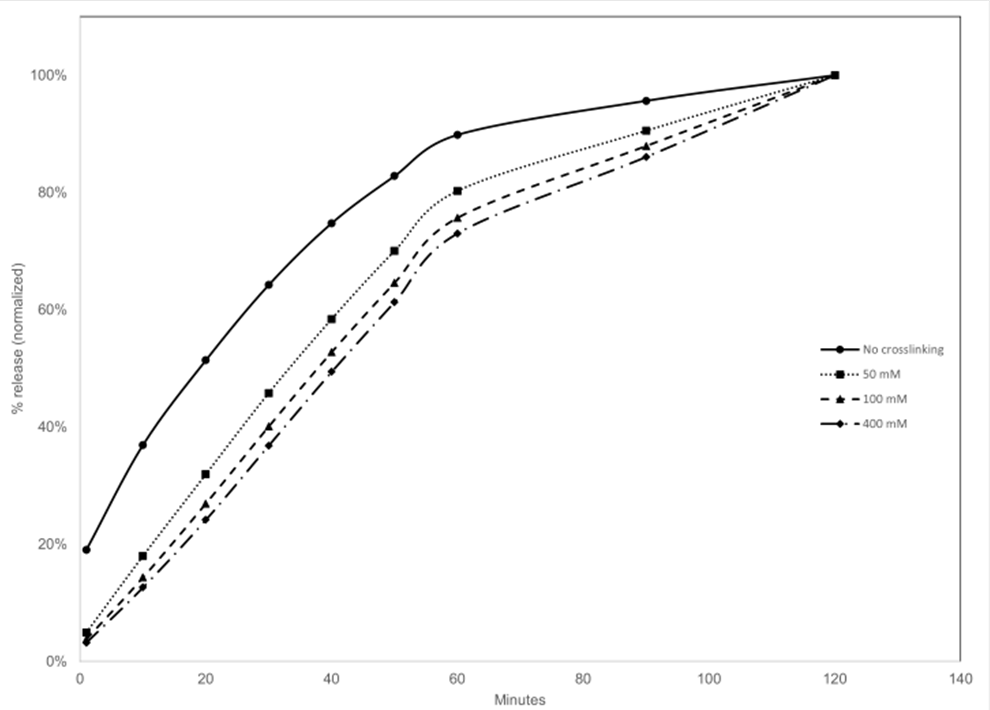
Methods: 3” silicon wafers are spin coated with an aqueous sodium alginate/budesonide suspension and crosslinked with CaCl2 (0, 50, 100, 400 mM). Drug release into simulated saliva from 3 cm square film was measured via HPLC. Drug release studies were repeated using identical 3x3cm films cut into small films 300-500 um2.
Results: All films showed sustained release in predictable fashion based on concentration of calcium chloride. The drug is released near linearly in the first 60 minutes (Figure 1). Cutting the larger films into small films resulted in an increased rate of release, but the overall profile of tunable sustained release is maintained.
Conclusion: Here we demonstrate a tunable, sustained release form of oral budesonide designed to prolong residence time in the esophagus. Stability studies are currently being performed. Next steps will include evaluating residence time versus conventional budesonide delivery.
Normalized release of budesonide from alginate films over 120 minutes