Pharmacometrics & Pharmacokinetics (PMK)
Poster Session II
LB-008 - DEVELOPMENT OF HOLISTIC EXPOSURE-TOXICITY RELATIONSHIPS FOR MMAE-BASED ADCS: A GENERALIZED PK/PD MODEL.
Thursday, March 23, 2023
5:00 PM - 6:30 PM EDT
H. Chang, Y.K. Cheung, D. Shah; The State University of New York at Buffalo, Buffalo, NY, United States.

Hsuan Ping Chang (she/her/hers)
PhD Candidate
University at Buffalo
Buffalo, New York, United States
Presenting Author(s)
Background: A generalized exposure-response (E-R) relationship is needed to support clinical pharmacology strategy of MMAE-based ADCs. We aimed to establish a generalized pharmacokinetic/pharmacodynamic (PK/PD) model to characterize PK and safety data of vc-MMAE ADCs in clinics.
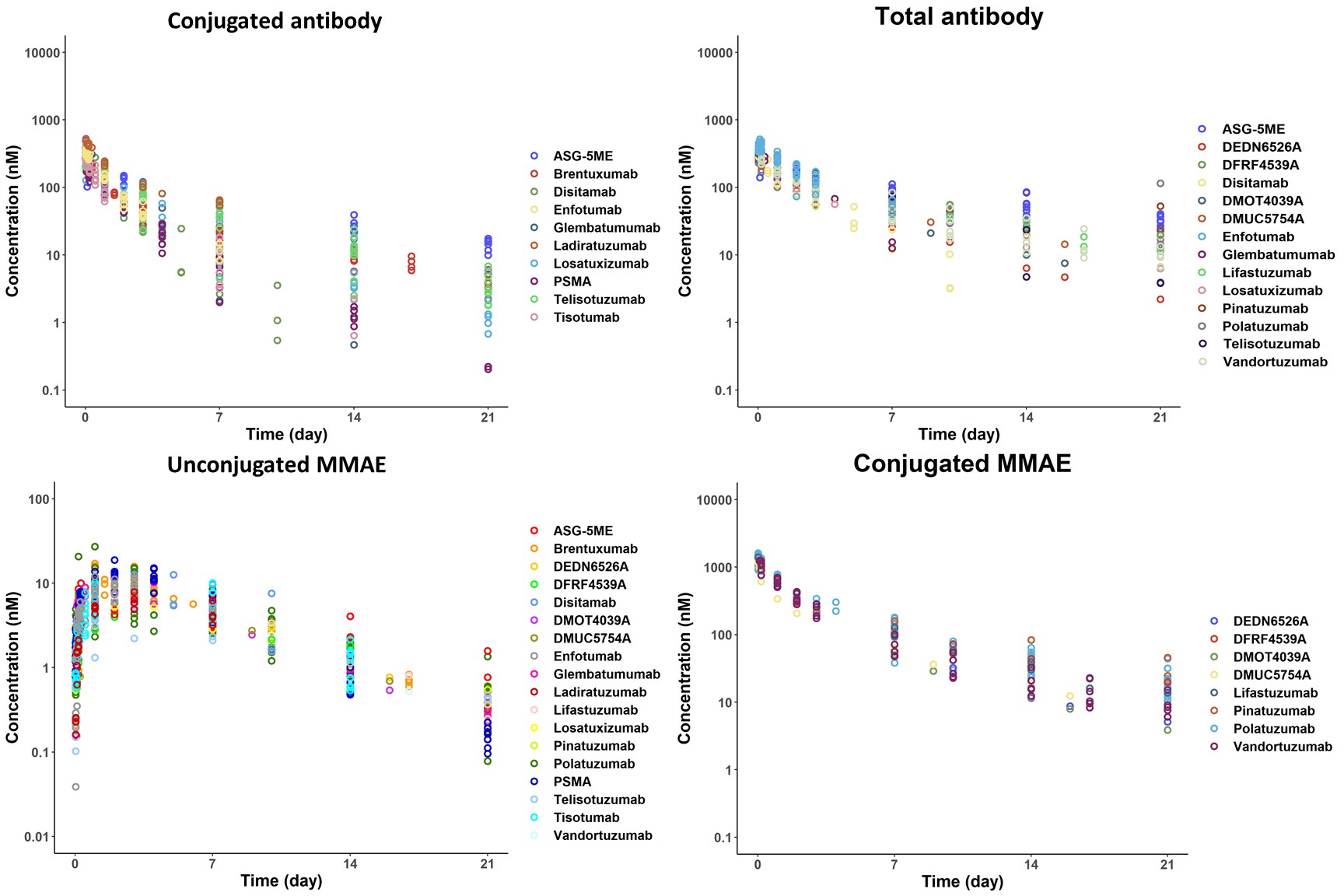
Methods: PK data of 4 ADC analytes (conjugated/total antibody, unconjugated/conjugated MMAE) for 18 MMAE-based ADCs were collected in 34 clinical studies from literature. Data were dose-normalized and compiled to assess the generalizability of clinical PK of MMAE-based ADCs. A deterministic PK model was developed to simultaneously capture PK of 4 ADC analytes. Toxicity endpoints were collected, and a PK/PD model was developed to characterize the E-R relationships of MMAE-based ADCs.
Results: Dose-normalized PK profiles of 4 ADC analytes were remarkably similar, confirming MMAE-based ADCs PK were generalizable (Figure). Our model characterized PK of 4 ADC analytes well across different targets, indications, and dosing, with > 93% data within 90% prediction interval. PK of conjugated MMAE as PD driver enables PK/PD model capturing peripheral neuropathy and neutropenia toxicity observations.
Conclusion: The generalized PK/PD model for MMAE-based ADCs enables a priori prediction of E-R relationships of novel ADCs with similar linker-payload while requiring further validation when applying to other ADC platforms.
 >
>
Methods: PK data of 4 ADC analytes (conjugated/total antibody, unconjugated/conjugated MMAE) for 18 MMAE-based ADCs were collected in 34 clinical studies from literature. Data were dose-normalized and compiled to assess the generalizability of clinical PK of MMAE-based ADCs. A deterministic PK model was developed to simultaneously capture PK of 4 ADC analytes. Toxicity endpoints were collected, and a PK/PD model was developed to characterize the E-R relationships of MMAE-based ADCs.
Results: Dose-normalized PK profiles of 4 ADC analytes were remarkably similar, confirming MMAE-based ADCs PK were generalizable (Figure). Our model characterized PK of 4 ADC analytes well across different targets, indications, and dosing, with > 93% data within 90% prediction interval. PK of conjugated MMAE as PD driver enables PK/PD model capturing peripheral neuropathy and neutropenia toxicity observations.
Conclusion: The generalized PK/PD model for MMAE-based ADCs enables a priori prediction of E-R relationships of novel ADCs with similar linker-payload while requiring further validation when applying to other ADC platforms.
 >
>