Back
Physiological Based Pharmacokinetic Modeling and Simulation (PBPK)
Poster Session II
LB-014 - POPULATION PHARMACOKINETIC ANALYSIS OF PHASE 1 SUBCUTANEOUS LEVOTHYROXINE FORMULATION (XP-8121).
Thursday, March 23, 2023
5:00 PM – 6:30 PM EDT
D. Mould1, R. Fitch2, R. Huang2, D. Harper2; 1Projections Research, Inc, Phoenixville, PA, United States, 2Xeris Pharmaceuticals, Chicago, IL, United States.

Robbie Huang, MS
Director, Biostatistics and Statistical Programming
Xeris Pharmaceuticals
Plainsboro, New Jersey, United States
Speaker(s)
Background: XP-8121 is a novel, subcutaneous (SC) injection formulation of levothyroxine that offers the potential to mitigate many of the dosing and absorption challenges associated with oral formulations. This analysis was conducted to develop a population pharmacokinetic (PPK) model to describe the concentration time profile of XP-8121 and use the model for simulations with different dosing scenarios.
Methods: Nonlinear, mixed-effect modeling (NONMEM) was used to analyze pharmacokinetic (PK) data. The final model was used to simulate 500 replicates using the design, dose regimen, and covariates of a Phase 1, randomized, open-label, crossover, bioavailability study of XP-8121 and oral levothyroxine (Synthroid®) in 60 healthy volunteers.
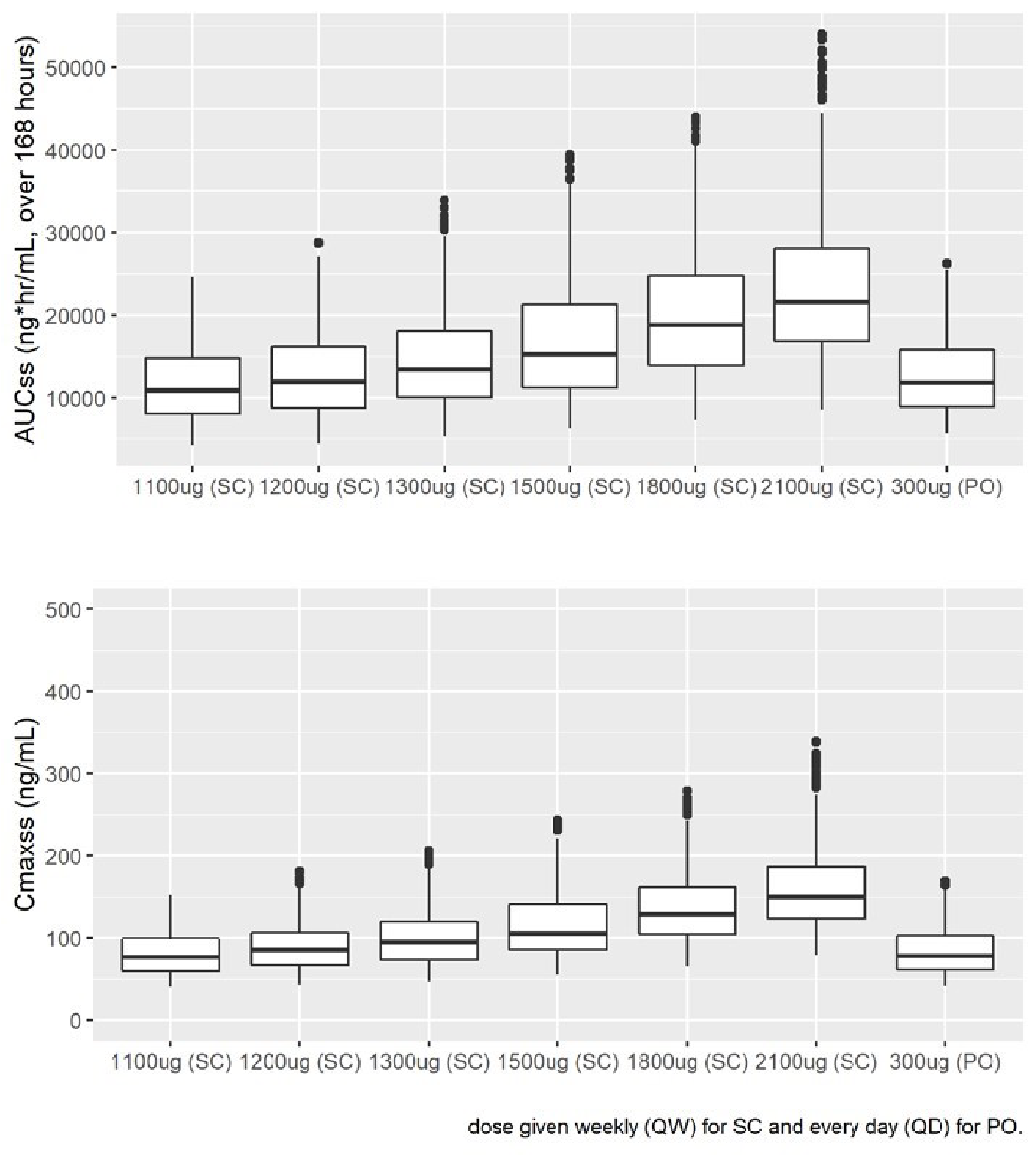
Results: XP-8121 is best described by a 1-compartment model with first-order elimination. Body weight (WT) was identified as a covariate on the clearance from central compartment (CL) and on central volume of distribution (Vc). XP-8121 (1200 µg SC) and Synthroid (300 µg PO) are similar in exposure at steady-state (AUC and Cmax) based on the simulations from the final PPK model (Figure).
Conclusion: The final PPK model provides adequate predictive performance for XP-8121 to inform future Phase 2 trials.
Methods: Nonlinear, mixed-effect modeling (NONMEM) was used to analyze pharmacokinetic (PK) data. The final model was used to simulate 500 replicates using the design, dose regimen, and covariates of a Phase 1, randomized, open-label, crossover, bioavailability study of XP-8121 and oral levothyroxine (Synthroid®) in 60 healthy volunteers.
Results: XP-8121 is best described by a 1-compartment model with first-order elimination. Body weight (WT) was identified as a covariate on the clearance from central compartment (CL) and on central volume of distribution (Vc). XP-8121 (1200 µg SC) and Synthroid (300 µg PO) are similar in exposure at steady-state (AUC and Cmax) based on the simulations from the final PPK model (Figure).
Conclusion: The final PPK model provides adequate predictive performance for XP-8121 to inform future Phase 2 trials.

